Colleagues:
 On World Malaria Day, the U.S. President’s Malaria Initiative (PMI) released its Tenth Annual Report to Congress [PDF, 11.1MB] documenting progress against the mosquito-borne disease.
On World Malaria Day, the U.S. President’s Malaria Initiative (PMI) released its Tenth Annual Report to Congress [PDF, 11.1MB] documenting progress against the mosquito-borne disease.
With PMI support, hundreds of millions of people have benefited from protective measures and have been diagnosed and treated for malaria. PMI has reached into the poorest of communities in sub-Saharan Africa, where malaria flourishes, arming women, children, and families with tools to protect themselves from malaria and providing them with fast-acting medicines to treat malaria if they do become infected.
The efforts of PMI have paid off. Working with host-country governments, donor governments, multilateral agencies, non-governmental organizations, and academic and research partners, 6 million deaths have been averted. In PMI focus countries, we witnessed reductions in both death and illness from malaria. Some of those countries are now setting their sights on eliminating malaria transmission from all or part of their nations, an idea that was inconceivable 10 years ago when PMI was launched.
We must continue to work to reverse the misfortune that conspires against a child’s survival, like being born too far from health services, too poor to buy life-saving commodities, seen by inadequately trained health care workers, surrounded by cultural norms, and practices that clash with modern science.
Roll Back Malaria Board
The intensified, collaborative effort by Roll Back Malaria (RBM) partners to support affected countries to end malaria is saving millions of lives, increasing attendance at school, improving worker productivity, and boosting local economies. But, malaria remains a serious public health threat. Eliminating malaria is critical to achieving the Sustainable Development Goals and must remain a key priority for global development community.
In December 2013, RBM commissioned an External Evaluation to ensure the Partnership remained fit-for-purpose to drive continued momentum toward a malaria-free world. The evaluation highlighted that significant adjustments to RBM’s structure would be necessary to sustain its successes and build on them to deliver on the WHO Global Technical Strategy (GTS) and accompanying RBM Action and Investment to defeat Malaria (AIM) ambitious 2030 goals and objectives.
In December 2015, the RBM Board unanimously approved a recommendation to transition the RBM Partnership to new governance structures to move it conclusively into a new era as we strive toward a world free of malaria.
More than 100 nominations for the Partnership Board were received from around the world. After a robust assessment and selection process, 13 individuals have been selected to take the revitalized Partnership forward.
Last month, the new board met face-to-face for the first time. I applaud the courageous and principled decision by the previous board and welcome new members. And, I am honored to serve on the new board alongside:
- Dr. Winnie Mpanju-Shumbusho, former Assistant Director General – Malaria, HIV, TB, NTDs, WHO; and new RBM Partnership Board Chair
- Mr. Kieran Daly, Deputy Director: Global Policy & Advocacy – Malaria, HIV, TB and the Global Fund, Bill & Melinda Gates Foundation, and new RBM Partnership Board Vice Chair
- Dr. Pedro L. Alonso, Director of the WHO Global Malaria Programme
- Mr. Elhadj As Sy, Secretary General, International Federation of Red Cross and Red Crescent Societies
- Mr. Simon Bland, Director – New York Office, UNAIDS
- Prof. Awa Coll-Seck, Minister of Health & Social Welfare, Senegal
- Mr. Paulo Gomes, Chairman, Paulo Gomes and Partners, former Executive Director, World Bank
- Dr. Richard Nchabi Kamwi, Elimination 8 Ambassador, former Minister of Health, Namibia
- Dr. Altaf Lal, Senior Advisor on Global Health and Innovation, Sun Pharmaceuticals Industries
- Mr. Ray Nishimoto, President of Health & Crop Sciences Sector, Sumitomo Chemical
- Dr. David Reddy, Chief Executive, Medicines for Malaria Venture
- H.E. Yongyuth Yuthavong, former Deputy Prime Minister and former Minister of Science and Technology
Call Your Shot
 Prior to World Malaria Day, basketball superstar Stephen Curry launched Call Your Shot to beat malaria challenge when he called – and then made – a trick shot and challenged others to take their shot to end malaria. To learn more about the campaign and to get involved, please go to USAID’s site at www.usaid.gov/endmalaria or to the campaign’s site at www.CallYourShot.org.
Prior to World Malaria Day, basketball superstar Stephen Curry launched Call Your Shot to beat malaria challenge when he called – and then made – a trick shot and challenged others to take their shot to end malaria. To learn more about the campaign and to get involved, please go to USAID’s site at www.usaid.gov/endmalaria or to the campaign’s site at www.CallYourShot.org.
Since the launch, sports stars like Charles Barkley and Shaquille O’Neal, business leaders like Facebook’s Sheryl Sandberg, and everyday champions have recorded videos of themselves calling out and making a shot to signal their support for ending malaria. The campaign has gained traction in more than 100 countries and has made hundreds of millions of impressions on social media.
The Call Your Shot challenge builds on Steph’s legacy of service as a cultural ambassador and Nothing But Nets Champion. I commend him for his efforts to inspire, educate, and mobilize a movement to reduce the devastating burden of malaria.
U.S. Government Malaria Research
Research to support malaria control efforts and reduce the burden of malaria has been a high priority of the U.S. Government for many years. The U.S. Government effort involves U.S. Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), Department of Health and Human Services (HHS), the Naval Medical Research Center (NMRC), and the Walter Reed Army Institute of Research (WRAIR) of the U.S. Department of Defense (DOD), and the U.S. Agency for International Development (USAID). Each of these agencies has its own direct funding for malaria research, and PMI funds operational research in addition to these other research efforts.
PMI investments in operational research play an important role in supporting the successful implementation of PMI prevention and treatment measures and in achieving PMI goals. This research focuses on program-relevant questions, complementing the more upstream vaccine and drug development work funded by NIH, DOD, and USAID.
As new or improved malaria interventions are developed and efficacy is demonstrated, operational research will be required to see how best to incorporate those new interventions into malaria activities, including understanding how well and under what conditions these tools work, the best and most cost-effective combination of tools, and whether certain interventions can be tailored to specific ettings.
RTS,S Vaccine
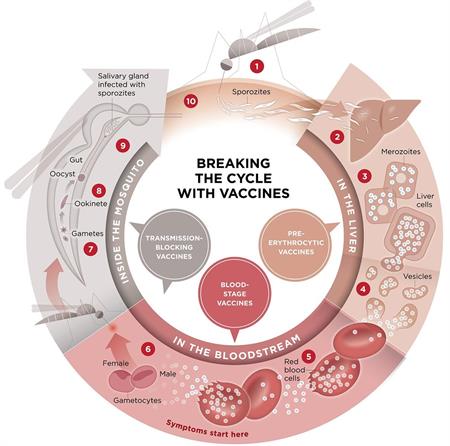 For almost a century, people have sought a malaria vaccine. Vaccines save millions of lives each year. Immunization has led to the eradication of smallpox, a significant reduction in childhood deaths from measles, and the near-eradication of polio. But a vaccine to protect against Plasmodium falciparum, the most deadly malaria parasite globally, and the most prevalent in Africa, has been more elusive.
For almost a century, people have sought a malaria vaccine. Vaccines save millions of lives each year. Immunization has led to the eradication of smallpox, a significant reduction in childhood deaths from measles, and the near-eradication of polio. But a vaccine to protect against Plasmodium falciparum, the most deadly malaria parasite globally, and the most prevalent in Africa, has been more elusive.
In June, Gavi, the Vaccine Alliance, agreed to support pilot testing of RTS,S – the first candidate vaccine for the prevention of malaria to be approved for pilot testing in moderate to high malaria transmission settings in 3-5 sub-Saharan African countries – contingent on matching funding. WHO plans to assess how the world’s first malaria candidate vaccine might be used alongside other tools to prevent malaria. The vaccine is being considered as a complementary malaria control tool in Africa that could potentially be added to – and not replace – the core package of proven malaria preventive, diagnostic, and treatment interventions. PMI is committed to keeping intervention coverage at scale in pilot countries. See the WHO press release and its Questions and Answers on RTS,S.
RTS,S aims to trigger the body’s immune system to defend against the Plasmodium falciparum malaria parasite when it first enters the human host’s bloodstream and/or when the parasite infects liver cells.
USAID, as well as NIH, CDC, and the Military Malaria Vaccine Program through WRAIR, and NMRC of DOD, contribute to the development of safe and effective malaria vaccines. The USAID malaria vaccine program has three major multi-year thrusts, two of which are (1) efforts targeting the parasite blood stage (the stage that is responsible for illness) and (2) efforts targeting the liver stage (the precursor to the blood stage). The third thrust is a vaccine that stimulates an immune response at the point the parasite enters the human bloodstream after a mosquito bite.
The good news is that the scientific community is already working to improve upon the achievement of RTS,S. Not only is there a strong program to improve RTS,S, but also there are multiple programs addressing all of the approaches above, not only in the U.S. Government but in universities and industry in the United States and Europe. There are widely different estimates of when new vaccines will be ready for advanced development. In addition to its technical development, any vaccine needs to be available, efficacious, cost effective, and safe; it must clear all regulatory hurdles; and countries must choose if, when, and where to use this new tool in addition to the proven-effective tools that exist now.
Respectfully,

Tim
R.T. Ziemer
Rear Admiral, U.S. Navy (ret)
U.S. Global Malaria Coordinator
